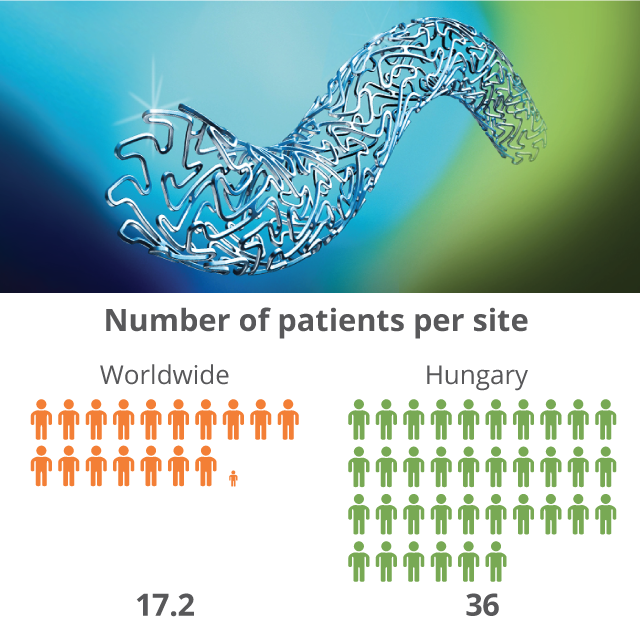
Medical device trials
and Consultancy
Our experienced and specialized team helps take medical device designs from concept to market. HungaroTrial provides full-service clinical trials with regulatory consulting for MDR compliance and CE Mark acquisition.
We are devoted to effective collaboration with global innovators, developing new ways to improve patient lives. Many of our CRAs and Project Managers are dedicated to specific sites and investigators and have worked closely with them for over a decade. Accordingly, they know which sites and investigators are best suited to ensuring quality and rigor as you navigate clinical development.



Study Concept Consultation
To support your clinical trial program’s conceptualization and development, our experienced management team will help you optimize study design for care standards and communication with KOLs and Notified Bodies. Additionally, we will help you assess feasibility, risks, and opportunities for scientific integrity, subject protection, regulatory compliance, and performance. We also help validate concepts and propose adaptive alternatives, where required.
Protocol Writing
We help ensure your Investigational Plan is suitable for Regulatory Bodies, ethically acceptable, easy to implement, easy for investigational staff to comply with, and adequate for patient recruitment and retention. Our resident physicians possess the medical writing skill, research experience, and records management background to effectively translate study concepts into ISO 14155-compliant plans, complete with supporting operational and technical instructions.
Trial Site Selection
HungaroTrial’s presence in many European countries has allowed us to establish over 1500 clinical trial sites with a history of excellent recruitment, compliance and performance, while our reputation allows us to form productive relationships with many KOLs. We arrange pre-study consultations and welcome each client’s active involvement in the selection process. This helps us quickly reach a mutual understanding regarding requirements, technical prerequisites, and expectations for trial conduct.
Clinical Trial Application
HungaroTrial has created a medical device regulatory intelligence system that helps us navigate complex regulatory frameworks with minimal friction. We faciliate direct contact and consultation with Competent Authorities, ensuring timely applications and reporting. Centralized, expert review of technical documentation assures validation for each submission, and high-touch coordination with each project team aligns daily operations to regulatory strategy.
Clinical Trial Project Management
By aligning operations, and project management, we harness the full knowledge, clinical research experience, and functional skills of our highly qualified medical personnel. Our project management team plans, implements, organizes, and communicates at every level and functional domain, executing effectively within the constraints of scope, quality, budget and time. This, in turn, minimizes risk and ensures efficient issue resolution.
Device Logistics Center (DLC)
The role of the Device Logistics Center (DLC) is to plan, direct, monitor, track and promptly resolve issues along the supply chain and throughout a clinical trial’s lifecycle. DLC advises and supports local purchases, interacting directly with authorized distributors. DLC intensively monitors critical sequences (transit time, import/export time, and delivery), as well as the implementation of effective measures for secure, controlled, and traceable transport and storage.
Device Service Support Center
Device operability is vital for trial quality and performance when technology is the object of investigation. Our Device Service Support Center ensures consistent device use according to user manuals, provides product related technical and service background knowledge, facilitates communication between trial sites and clients, and troubleshoots issues in a prompt, user-friendly fashion.
Device Vigilance Management
Depending on the stage of clinical investigation, class, type of medical device, and risk clarity, HungaroTrial provides the resources, organization, management, and validated systems for identifying, evaluating and reporting adverse events. Our goal is the facilitation of timely interaction, as well as the exchange of relevant information between clientss and relevant regulatory authorities.
Data Management and Statistics
Maintaining data integrity throughout a clinical trial’s lifecycle has always been a crucial component of regulatory assessment. In recent years, this endeavour has been challenged by digitalization, virtualization, and increasing scrutiny around security, confidentiality, and long-term data retention. To that end, HungaroTrial offers validated, customizable, and compliant “input-to output” eSolutions. These solutions are capable of integrating biostatistics, designing and developing Case Report forms, and facilitating data validation, analysis, and reporting.
Clinical Trial Report Writing
We engage medical and scientific experts, statisticians, and regulatory reviewers for scientific validity and regulatory relevance of CIR. Our skilled and experienced physician medical writers ensure CIR is prepared with content, format, and structure aligned to ISO 14155. From our perspective, preparation and submission of CIR is a project with defined structure, responsibilities, timelines, and deliverables, managed and coordinated by regulatory experts.
Gap Analysis For Correct Compliance
We provide a proven, three-step gap analysis approach for compliance. First, our regulatory and quality management experts review available technical documentation. Then, you receive a Gap Report highlighting your state of compliance with MDR and/or ISO 13485 requirements. Based on these findings, we develop a proposal to help you remediate gaps and create additional momentum around existing strengths.
Building Up Regulatory Strategy
HungaroTrial understands that regulatory clearance represents a pivotal stage of a medical device’s life cycle, from both business and clinical development perspectives. Accordingly, we help clients navigate the complex and highly-scrutinized regulatory pathway, taking care to understand and factor our clients’ unique, specific needs. We are aware that keeping an already-launched product in the market under a continuously changing regulatory environment is challanging, so we are here to support this and make sure that everything is under control.
Technical Documentation Compliance
Maintaining adequate Technical Documentation is a crucial part of meeting expectations along the conformity assessment pathway. HungaroTrial’s scientific and technical expertise, competency in medical device trials, and regulatory intelligence allows us to confidently manage Biocompatibility And Safety Tests, Clinical Evaluations, Post-Market Clinical Follow-Up, Post-Market Surveillance and Risk Management steps to ensure Technical Documentation remains suitable for conformity declaration.
Initial Gap Analysis and Strategy Set Up
We consider the early involvement of HungaroTrial’s scientific, technical, and regulatory experts a key factor for successful development. Understanding a product’s design from regulatory, healthcare, end user, and future market perspectives is essential to create the most value possible. Thoroughly evaluating available documentation against strategic goals is the start of a successful regulatory path. From there, we select the relevant Notified Body, determine elements for conformity assessments, create a schedule, and identify and audit critical subcontractors.
Technical Documentation Development
HungaroTrial supports its clients in the establishment and maintainenance submission-ready technical documentation. We offer efficient combination of expert, medical writing, technical and administrative competence for:
- Establishing, structuring, assembling and formatting technical documentation according to requirements
- Ongoing and comprehensive compliance review for Essential Requirements
- Reviewing clinical data and preparing a clinical evaluation report
Pre-audit Before Starting the Certification Process
One of HungaroTrial’s special advantages is the high availability of our Quality Management System professionals: personnel possessing in-depth knowledge and working experience with the evaluation of compliance with requirements and standards (such as MDD/MDR, ISO13485). HungaroTrial’s medical device specialists work with clients to determine pre-audit programming for certification. Audit programs may vary by scope and objectives, but they always focus on high-risk areas and opportunities to identify effective corrective actions and mitigations.
Supporting Technical Document Review
HungaroTrial applies streamlined and expedited procedures that ensure prompt, timely, and qualified responses to requests arriving from Notified Bodies during the Technical Documentation review. Qualified team members are responsible for analyzing questions and issues and providing clients with appropriate corrective actions. A final review prior to submission ensures all issues raised by a Notified Body are addressed completely and efficiently.
Conformity Assessment Process
HungaroTrial regulatory specialists contribute to inspection readiness and are at your disposal to help prepare you for audits initiated by Notified Bodies. We support communication with Notified Body auditors and facilitate responses, as needed, and have important expertise supporting the identification and implementation of corrective and preventive actions. These may include additional audits in areas with identified issues, to better evaluate and define actions in line with root-cause.
Post-Market Activities
After a successful product launch and certification, HungaroTrial’s expert team will help ensure continued compliance throughout a launched product’s lifecycle. We align towards compliance in a product’s early stages, saving time and reducing client expenses.
Post-Market Clinical Follow-Up isfrequently challenging. HungaroTrial’s Regulatory Services team can fully support these activities, including PMCF studies and the collection and review of PMCF Clinical Data.




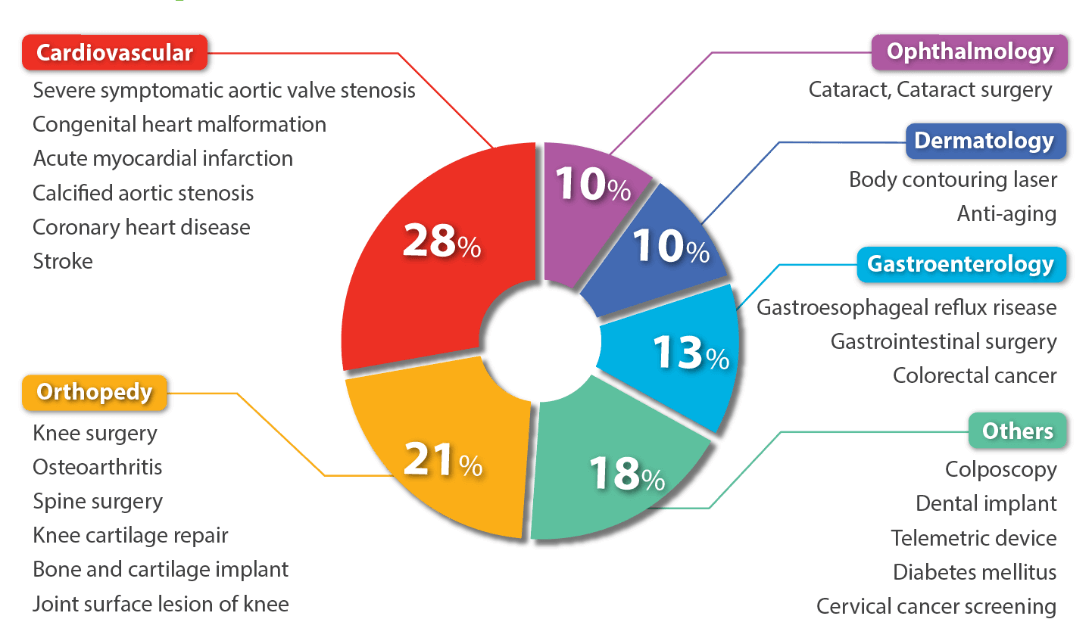
Therapeutic areas
We offer services across a broad spectrum of therapeutic areas, including Cardiovascular, Orthopedy, Gastroenterology and many others. Our experience lets us facilitate the complex clinical trials and provide customized solutions for medical device manufacturers.
Selected experience list
Director of Medical Device Unit

Antal Solyom, MSc
14 years at Notified Body TUV Rheinland as Medical Device Expert
- 4 years as Lead Auditor and Medical Device Expert
- 2 years as LA in TUV Rheinland of North America in USA
- 8 years as Certifier and Head of Certification Office
3 years at Notified Body ORKI as Medical Device Expert and LA
Graduated as Mechanical Engineer MSc at University of Miskolc
Contact our Medical Device Team at
Advance Your Program Today
HungaroTrial is ready to help you complete your clinical trial program on time and to the highest standards.
Clinical development / Phase I-IV. services
We provide exceptional study design, management, monitoring, and reporting expertise for our partners to support their confident decisions for successful clinical development. Our network in the medical community of the CEE Region and long-term KOL connections ensure optimal trial site selection and enrolment rates for our Clients.
CRA Outsourcing
For over 20 years HungaroTrial has provided CRA sourcing services in most CEE countries. The best proof of our service’s quality is the long list of our repeat clients, including 7 of the TOP 10 global pharmaceutical companies.
Support for COVID-19 research
From the beginning of the pandemic, HungaroTrial has been very active in COVID-19 clinical research. Our colleagues are dedicated to add our clinical development knowledge, medical expertise and research infrastructure to the global efforts to find safe and effective COVID-19 medications.