World-Class
Clinical Trial Services
HungaroTrial is a regional Contract Research Organization (CRO) based in Central and Eastern Europe. Since 1999, we have helped healthcare organizations around the world – including pharmaceutical, biotechnology, and medical device companies – drive effective clinical development. Whether you need to develop a strategic plan that assures success, create robust trial processes, understand regional nuance, accelerate program development, or effectively manage risk over a project’s entire lifecycle, HungaroTrial is at your service.






CRO Leadership in
Central & Eastern Europe
Our strong regional heritage, dedicated senior staff, and unique service model help you overcome key challenges associated with clinical trials, including recruitment, site management, rescue studies, and more.
As your dedicated partner and project manager, we help you rapidly start and scale programs, guarantee patient enrollment, and complete projects on time for all trial phases and major therapeutic areas, including but not limited to cardiology, oncology, rheumatology, rare disease, CNS and respiratory.
Learn more about usAdvance Your Program Today
HungaroTrial is ready to help you complete your clinical trial program on time and to the highest standards.
Why HungaroTrial?
Experienced, Engaged Medical Staff
Superior Regional Presence
Rapid Start – Up and Strict Timeliness
Patient Enrolment Confidence
Robust Quality Management





Locations
HungaroTrial is a Regional CRO with the widest coverage in Central and Eastern Europe (CEE). With local offices in ten countries we are the most-connected and experienced CRO of this region. In 2023 we have expanded our clinical operations into the United States and opened an office in Atlanta. We are establishing a Business Development office in South Korea in 2024.
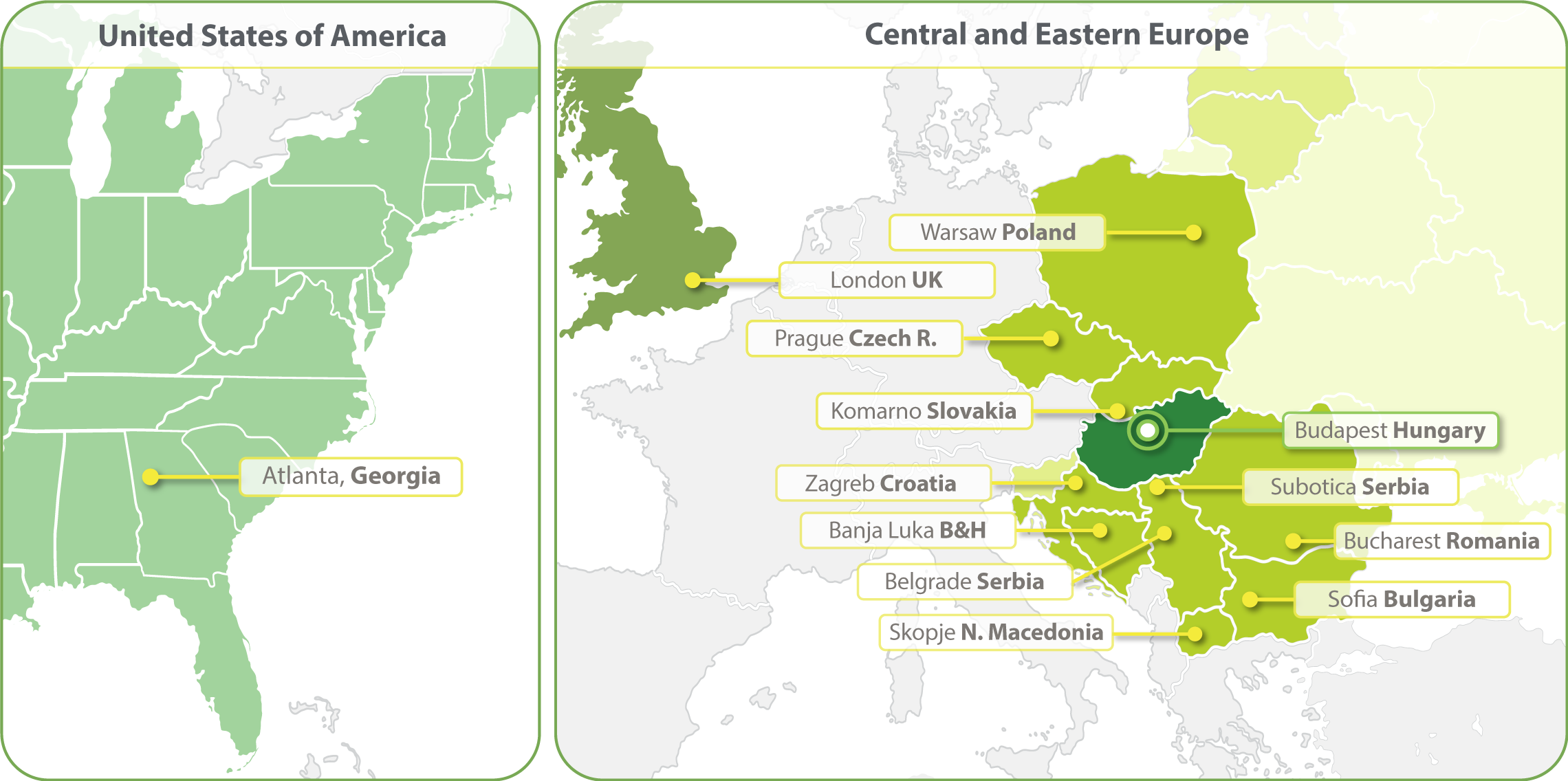
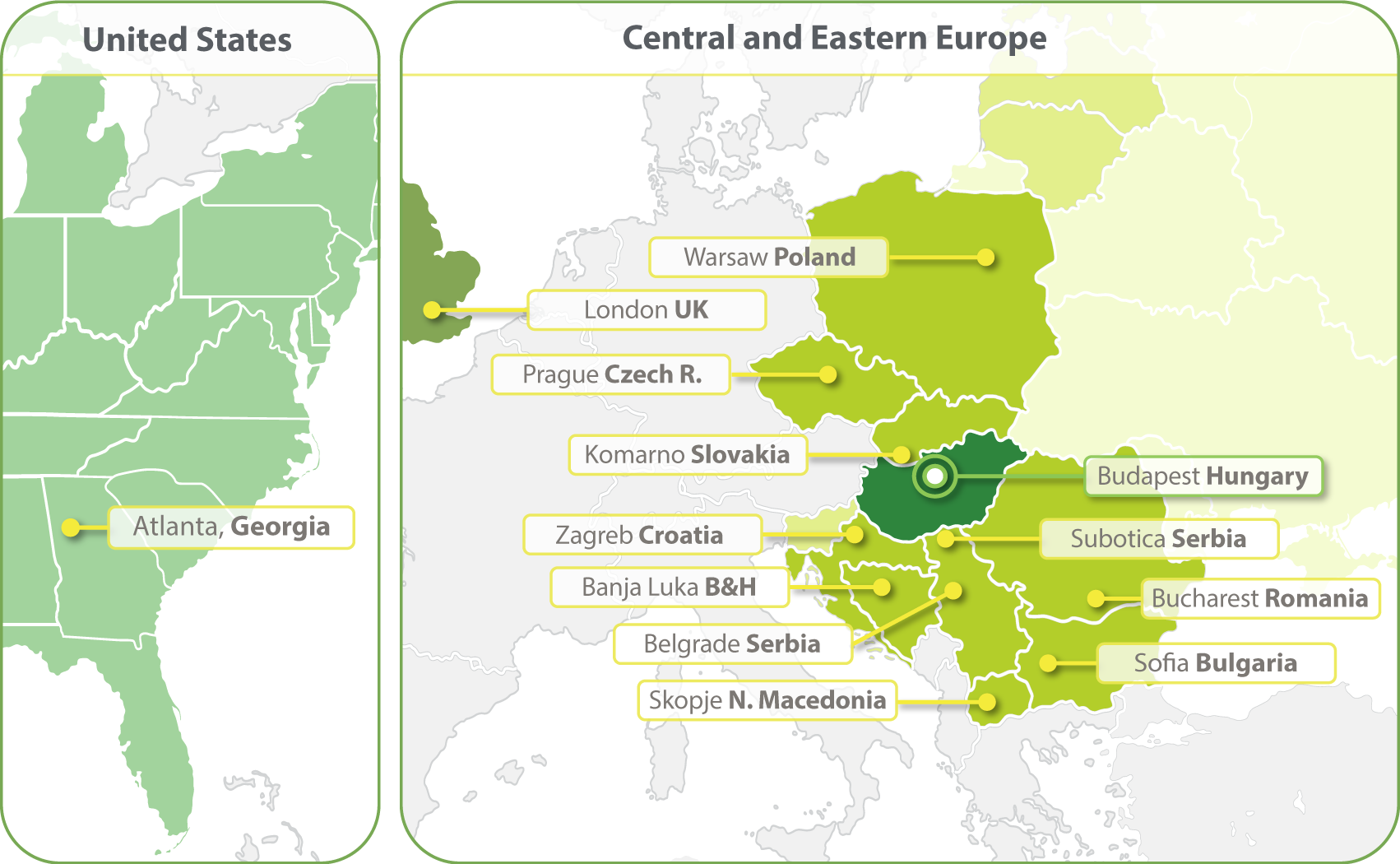
- Budapest, Hungary
- Zagreb, Croatia
- Banja Luka, Bosnia and Herzegovina
- Belgrade, Serbia
- Bucharest, Romania
- Komarno, Slovakia
- Skopje, North Macedonia
- Sofia, Bulgaria
- Subotica, Serbia
- Prague, Czech Republic
- Warsaw, Poland
- Estonia
- Georgia
- Latvia
- Lithuania
- Slovenia
- London, United Kingdom
- Seoul, South Korea
- Atlanta, Georgia
Management team


Dr. Sárosi is the CEO and founder of HungaroTrial. Since 1999, he has built the company to become the market leading regional CRO in Central and Eastern Europe. He is a recognized expert in the conduct of clinical studies in CEE and is a frequently invited lecturer at graduate and postgraduate programs and industry events such as the well-known BIO International Convention (USA).
Dr. Lajos Sarosi is a graduate of Semmelweis University Medical School, Budapest. He also received his postgraduate degree in 2018 after successfully completing the 2 years international CEMDC program inpharmaceutical medicine. While completing his medical degree, he moved to the USA to participate in a rotation at the State University of New York in Buffalo. After 2 years in medical practice, he joined the clinical research teams of Zeneca and Quintiles.

Lajos Sárosi, MD


Dr. Laszlo Nagy joined the HungaroTrial team in 2007 as our Director of Key Regional Research Projects. He is a graduate of Semmelweis University Medical School, Budapest. After completing his medical studies, Laszlo spent 8 years in medical practice as a rheumatologist. He is widely published in international journals on the field of immunology, including papers on systemic lupus erythematosus (SLE), Rheumatoid Arthritis and Psoriasis. In 1997, Dr. Nagy joined the clinical research team of Astra (later AstraZeneca), where he worked on international studies for the next 10 years. His publication list can be found here.

Laszlo Nagy, MD


Zsombor Matijevics is chemist with clinical research experience. He is a specialist of clinical trial conduct in the former Yugoslav countries. His deep knowledge in the regulatory, legal and logistical aspects of the clinical trials in the region is an essential tool for assuring fast study start up and successful trial management.

Zsombor Matijevics


Gabor Kucserka is an economist and received his MBA degree at Corvinus University, Budapest. He brings to the Company more than 20 years of experience leading financial operations, controlling, and overseeing IT projects. Prior to joining HungaroTrial, he worked in key financial positions for Wolters Kluwer and for Honda in Hungary and in Austria.
Mr. Kucserka is responsible for HungaroTrial’s group operations, including Finance, Accounting, Tax, Budgeting and Financial Planning, as well as all Information Technology functions.

Gabor Kucserka, MBA


Raluca Axinte is an economist by education and a detailed operations professional by nature. Ms. Axinte is responsible for the financial and technical aspects of HungaroTrial’s regional operations. She joined the company in 2005, and played an integral role in the start-up of our operations in Romania.

Raluca Axinte


Dr. Eva Reif graduated at Semmelweis Medical University, Budapest. She worked in medical practice for 10 years in the fields of Anesthesiology, Intensive therapy, and Cardiology. She obtained board Certification in Anesthesiology and Intensive therapy.
Later she decided to work in the Pharma Industry and spent 8 years as Clinical Research Associate at Bristol Myers Squibb, continued by 5 years as Clinical Project Leader at Sanofi – Aventis. She joined HungaroTrial as Clinical Research Manager and Country Manager in 2011.
Dr. Eva Reif is an expert in complex regulatory issues, has remarkable experience in managing medical device studies as well as protocol writing and reviews. She wrote 10+ clinical trial protocols in different indications, e.g. Oncology, Cardiology, Hypertension, Diabetes.

Éva Reif, MD


Adrienn Dusnoki is an economist and received her MBA degree at Corvinus University, Budapest. Since 2000, she has worked for HungaroTrial. In the first 4 years of her carrier at the company, she was the member of our clinical team, where she gained detailed knowledge about the clinical project management.
Since 2004, Adrienn has been responsible for Human Resource management functions, including selection, hiring our staff members, coordinating development and training, and maintaining detailed staff records.

Adrienn Dusnoki, MBA

Testimonials
“We hold a highly positive opinion of HungaroTrial CRO and strongly recommend any company looking for a CRO to initiate a quality study at the relevant geographical areas to consider HungaroTrial CRO’s services.”
“Having worked with many CROs over the last 10 years, I would rank HungaroTrial at the top of my list to work with.”
“HungaroTrial’s highly qualified staff is experienced, dedicated and professional. We never experienced any problems concerning the meeting of studies deadlines, as HungaroTrial has responded very quickly and efficiently to any request made. HungaroTrial has proven the ability to simultaneously manage clinical trials in three different countries.”
“I am convinced that HungaroTrial is a very reliable CRO, which offers high quality service. I strongly recommend them as a partner in clinical research.”
Advance Your Program Today
HungaroTrial is ready to help you complete your clinical trial program on time and to the highest standards.
