Clinical development /
Phase I-IV. services
We provide exceptional study design, management, monitoring, and reporting expertise for our partners to support their confident decisions for successful clinical development. Our network in the medical community of the CEE Region and long-term KOL connections ensure optimal trial site selection and enrolment rates for our Clients.


Protocol Design
HungaroTrial’s experienced Medical Team includes 12 medical doctors, as well as supporting pharmacists aand biologists. In addition, we are able to tap into a wide regional network of recognized clinical thought leaders (KOLs), forming the right team to help you navigate key clinical trial challenges, including protocol design. We analyze every aspect of your study project management plan, aligning scientific, ethical, and standard of care parameters to develop a study protocol that delivers excellence, preserves, transparency, and drives patient recruitment.
Medical Writing
Our medical writing team has extensive experience developing clinical trial assets, informed consent forms, study protocols, and investigator brochures, following all applicable ICH Guidelines and GCP requirements to the highest standard.
Regulatory Applications
HungaroTrial has decades of experience in Central and Eastern Europe clinical trial regulation. We have developed and refined an operatonal system that allows us to comply with dynamic, complex local regulations. We understand regulator expectations – both official and informal – and communicate with applicable national bodies quickly and efficiently. To that end, we have established a Local Regulatory Officer in each country of operation. The office is responsible for staying up-to-date on local regulation, training staff, and safeguarding the quality of clinical trial applications.
Clinical Operations
Clinical trial monitoring and site management services are provided by qualified, knowledgeable professionals with extensive clinical research and therapeutic area experience. Our CRAs deliver a spectrum of site management and monitoring functions, including trial site identification, qualification and selection, budget and contract negotiations, ongoing site monitoring and management, support for logistics, regulatory document collection, review and submission, and Electronic Trial Master File (eTMF) setup and maintenance.
Medical Monitoring
Clinicians with specific and relevant therapeutic experience drive every decision regarding a patient’s prospective enrollment, evaluating for eligibility and possible adverse events. Over time, we monitor trial data closely to proactively look for adverse events patterns, ensuring a high level of integrity as your patients progress through your clinical trial.
Pharmacovigilance
A qualified and experienced pharmacovigilance (PV) group is responsible for completing all safety reporting within the timeframe defined by local regulators. Additionally, our PV team carefully designs safety systems and protocols that safeguard patients from enlistment to completion.
Data Management and Statistics
We help drive crucial clinical trial data initiatives, including indexing, management, analysis, and final report creation. Specific functions include statistical analysis planning and reporting, sample determination, CRF design and correction, database design and development, database validation, data entry (simple/double), querie solving, codification and encryption.




Advance Your Program Today
HungaroTrial is ready to help you complete your clinical trial program on time and to the highest standards.
Medical device trials and Colsultancy
HungaroTrial’s Expert Team is ready to assist medical device companies through all phases of product development by regulatory consultancy and clinical trial services, with special focus on MDD => MDR transition.
CRA Outsourcing
For over 20 years HungaroTrial has provided CRA sourcing services in most CEE countries. The best proof of our service’s quality is the long list of our repeat clients, including 7 of the TOP 10 global pharmaceutical companies.
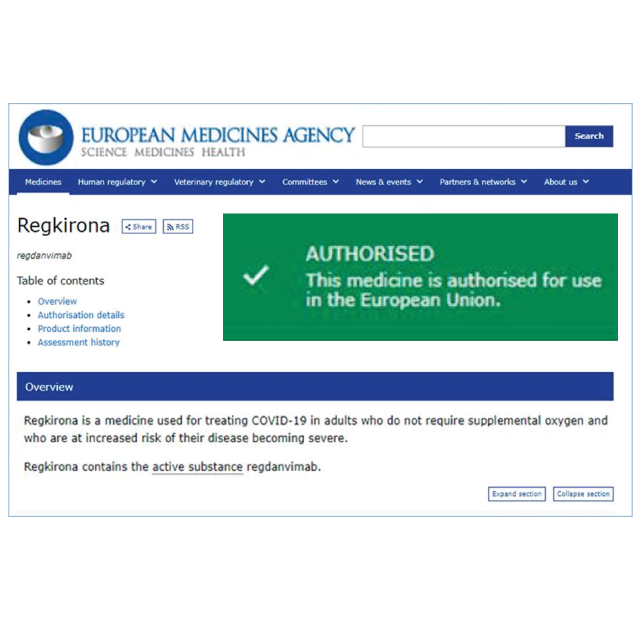
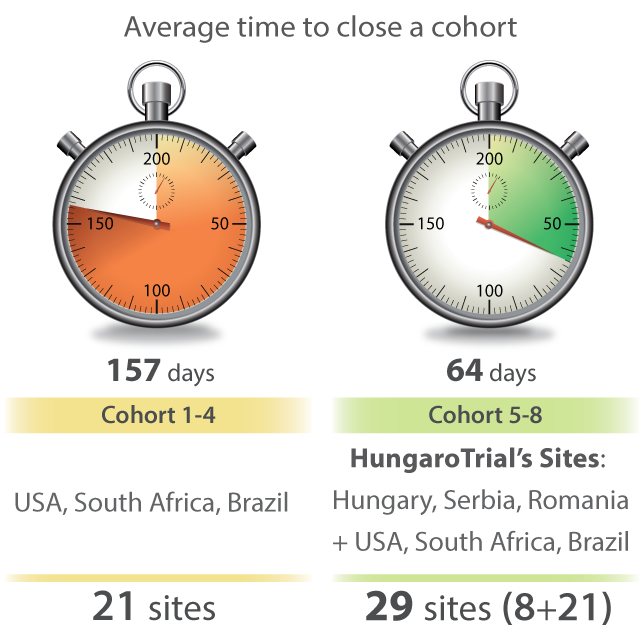
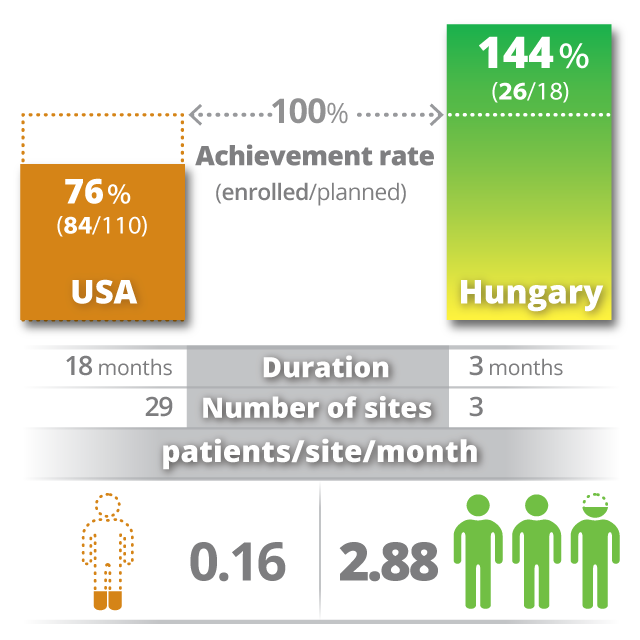
Support for COVID-19 research
From the beginning of the pandemic, HungaroTrial has been very active in COVID-19 clinical research. Our colleagues are dedicated to add our clinical development knowledge, medical expertise and research infrastructure to the global efforts to find safe and effective COVID-19 medications.