Company Profile
HungaroTrial is the market-leading clinical research organization (CRO) in the Central & Eastern Europe (CEE) region. We offer a comprehensive range of clinical trial development and support services for global healthcare organizations, including pharmaceutical, biotechnology, and medical device companies in all major therapeutic areas.
With 25 years of experience in clinical trial program design, operational consulting, and recruitment, we provide our clients the tools, knowledge, and expertise required to overcome key challenges, including patient enrolment, site management, and plan development.



History – A Legacy of Excellence
HungaroTrial is founded by Dr. Lajos Sárosi as CRO operating in the CEE region

Opens offices in Romania, Slovakia and Serbia

Begins operations in Poland and Bulgaria

Opens office in Bulgaria

Opens office in Czech Republic

Opens offices in Bosnia, North Macedonia and Poland

Opens office in Croatia

Performs first multi-country clinical trial

Expands reach to include Ukraine and Russia

Begins operations in Bosnia

Establishes Client Relations Office in London, UK

Begins operations in North Macedonia

US operations office opened

Management team


Dr. Sárosi is the CEO and founder of HungaroTrial. Since 1999, he has built the company to become the market leading regional CRO in Central and Eastern Europe. He is a recognized expert in the conduct of clinical studies in CEE and is a frequently invited lecturer at graduate and postgraduate programs and industry events such as the well-known BIO International Convention (USA).
Dr. Lajos Sarosi is a graduate of Semmelweis University Medical School, Budapest. He also received his postgraduate degree in 2018 after successfully completing the 2 years international CEMDC program inpharmaceutical medicine. While completing his medical degree, he moved to the USA to participate in a rotation at the State University of New York in Buffalo. After 2 years in medical practice, he joined the clinical research teams of Zeneca and Quintiles.

Lajos Sárosi, MD


Dr. Laszlo Nagy joined the HungaroTrial team in 2007 as our Director of Key Regional Research Projects. He is a graduate of Semmelweis University Medical School, Budapest. After completing his medical studies, Laszlo spent 8 years in medical practice as a rheumatologist. He is widely published in international journals on the field of immunology, including papers on systemic lupus erythematosus (SLE), Rheumatoid Arthritis and Psoriasis. In 1997, Dr. Nagy joined the clinical research team of Astra (later AstraZeneca), where he worked on international studies for the next 10 years. His publication list can be found here.

Laszlo Nagy, MD


Zsombor Matijevics is chemist with clinical research experience. He is a specialist of clinical trial conduct in the former Yugoslav countries. His deep knowledge in the regulatory, legal and logistical aspects of the clinical trials in the region is an essential tool for assuring fast study start up and successful trial management.

Zsombor Matijevics


Gabor Kucserka is an economist and received his MBA degree at Corvinus University, Budapest. He brings to the Company more than 20 years of experience leading financial operations, controlling, and overseeing IT projects. Prior to joining HungaroTrial, he worked in key financial positions for Wolters Kluwer and for Honda in Hungary and in Austria.
Mr. Kucserka is responsible for HungaroTrial’s group operations, including Finance, Accounting, Tax, Budgeting and Financial Planning, as well as all Information Technology functions.

Gabor Kucserka, MBA


Raluca Axinte is an economist by education and a detailed operations professional by nature. Ms. Axinte is responsible for the financial and technical aspects of HungaroTrial’s regional operations. She joined the company in 2005, and played an integral role in the start-up of our operations in Romania.

Raluca Axinte


Dr. Eva Reif graduated at Semmelweis Medical University, Budapest. She worked in medical practice for 10 years in the fields of Anesthesiology, Intensive therapy, and Cardiology. She obtained board Certification in Anesthesiology and Intensive therapy.
Later she decided to work in the Pharma Industry and spent 8 years as Clinical Research Associate at Bristol Myers Squibb, continued by 5 years as Clinical Project Leader at Sanofi – Aventis. She joined HungaroTrial as Clinical Research Manager and Country Manager in 2011.
Dr. Eva Reif is an expert in complex regulatory issues, has remarkable experience in managing medical device studies as well as protocol writing and reviews. She wrote 10+ clinical trial protocols in different indications, e.g. Oncology, Cardiology, Hypertension, Diabetes.

Éva Reif, MD


Adrienn Dusnoki is an economist and received her MBA degree at Corvinus University, Budapest. Since 2000, she has worked for HungaroTrial. In the first 4 years of her carrier at the company, she was the member of our clinical team, where she gained detailed knowledge about the clinical project management.
Since 2004, Adrienn has been responsible for Human Resource management functions, including selection, hiring our staff members, coordinating development and training, and maintaining detailed staff records.

Adrienn Dusnoki, MBA
Locations
HungaroTrial has expanded its operations to include clinical trial services in over 20 European countries, maintaining its core focus on Central & Eastern Europe.
Our strong regional clinical project management and regulatory competence ensures the services delivered to our clients are of the highest quality. In 2023 we have opened a clinical operations office in Atlanta.
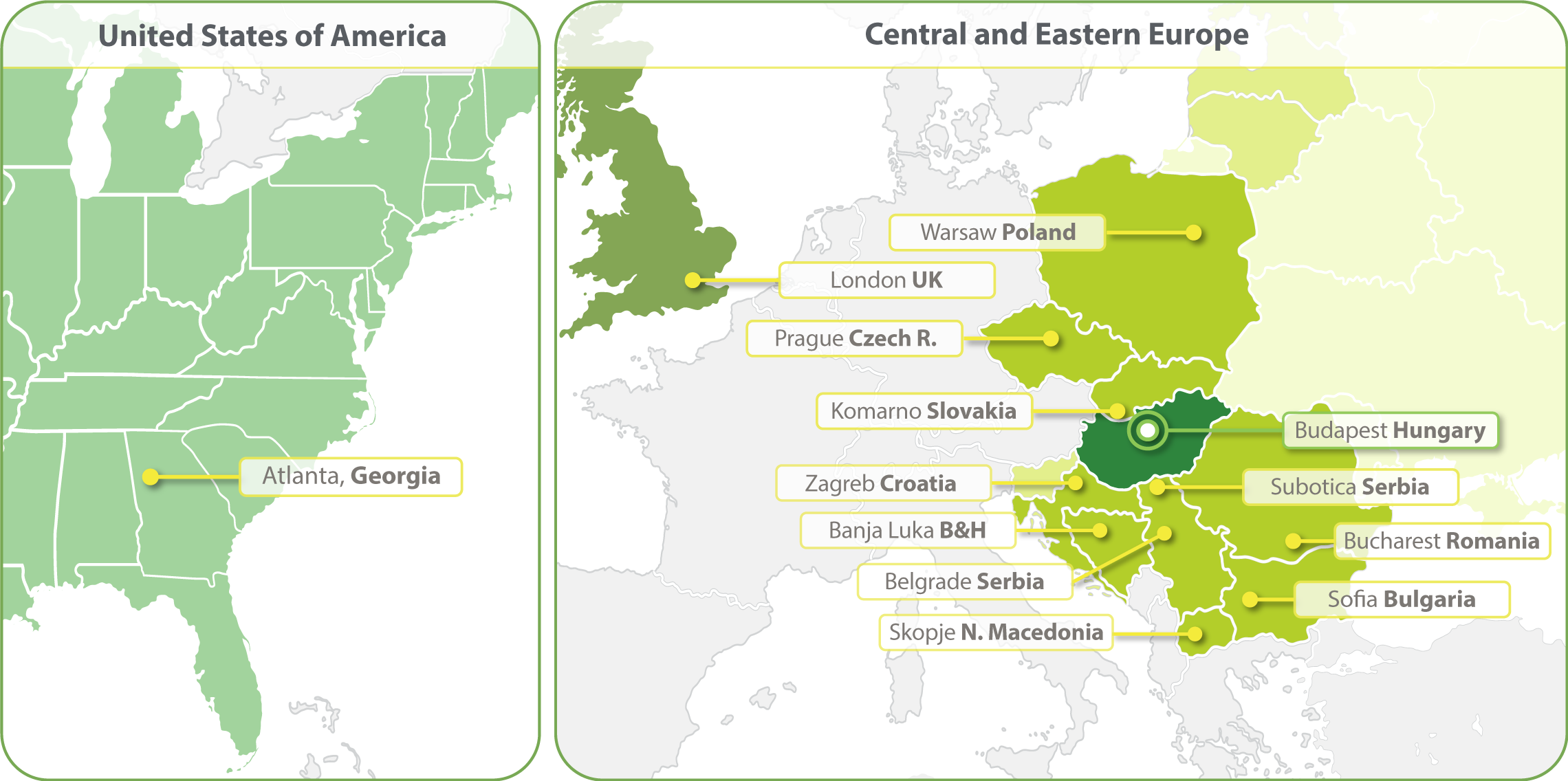
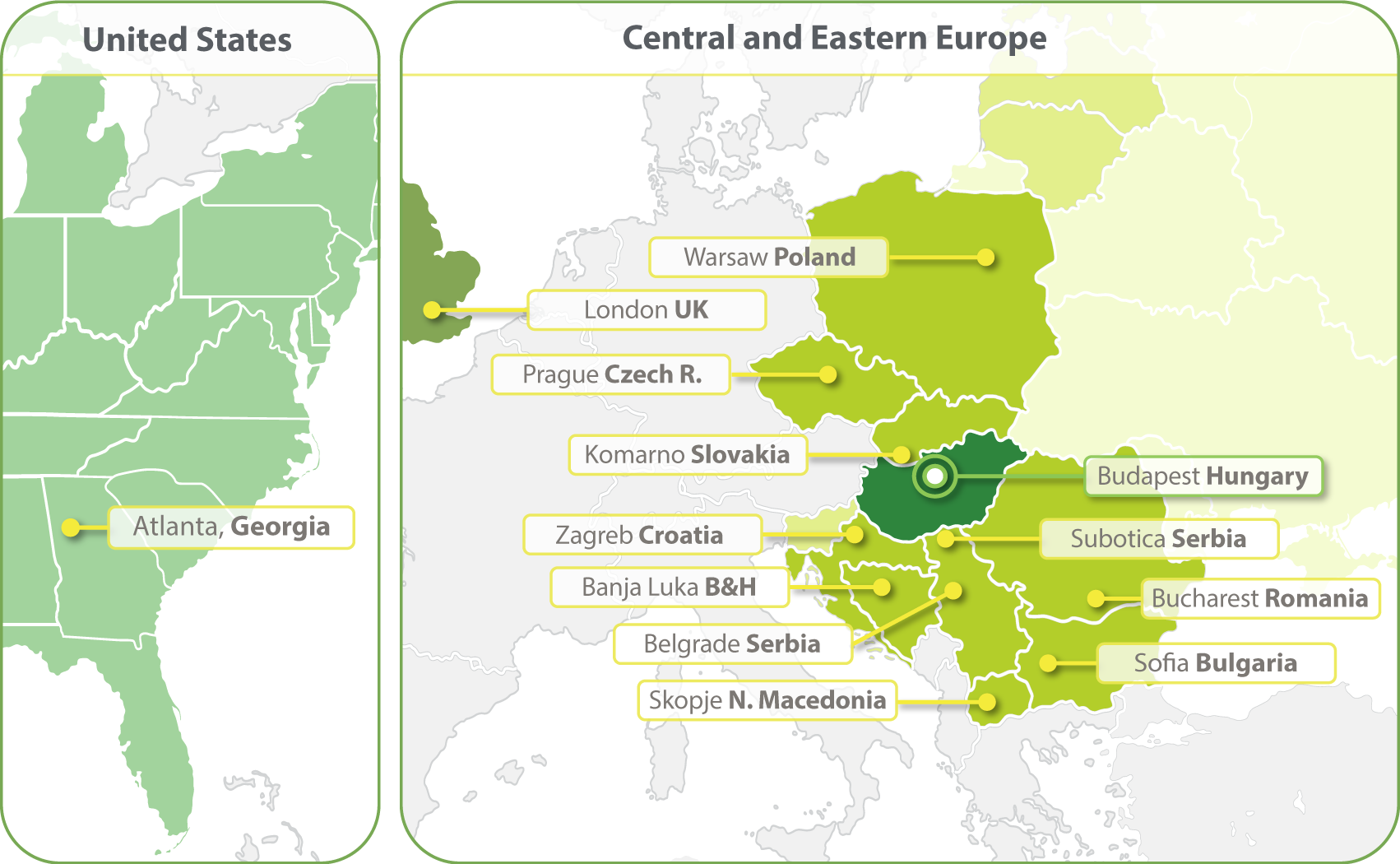
- Budapest, Hungary
- Zagreb, Croatia
- Banja Luka, Bosnia and Herzegovina
- Belgrade, Serbia
- Bucharest, Romania
- Komarno, Slovakia
- Skopje, North Macedonia
- Sofia, Bulgaria
- Subotica, Serbia
- Prague, Czech Republic
- Warsaw, Poland
- Estonia
- Georgia
- Latvia
- Lithuania
- Slovenia
- London, United Kingdom
- Atlanta, Georgia
Core Values
Experienced, Engaged Medical Staff
Superior Regional Presence
Rapid Start – Up and Strict Timeliness
Patient Enrolment Confidence
Robust Quality Management





Benefits Of Doing Clinical Trials In CEE
There are several important reasons to consider running your clinical trial in the CEE region, including:
Increased Productivity & Rapid Patient Recruitment
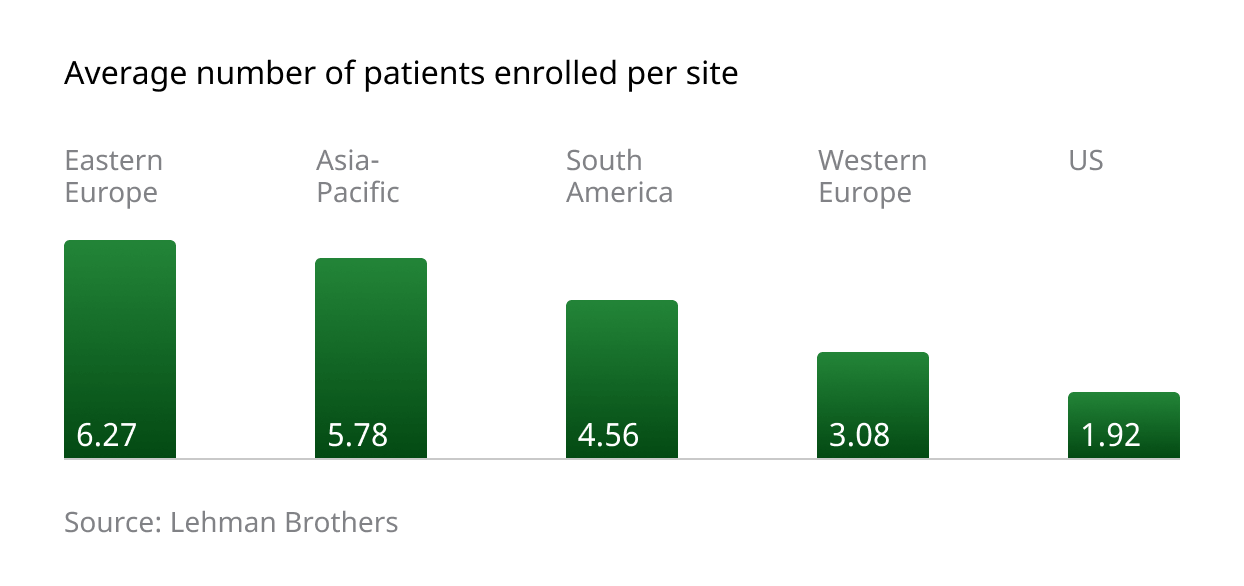
Patient enrolment figures in the CEE region are impressive, with a higher average number of patients enrolled per site (6.27) compared to competing regions:
Additionally, the region boasts a higher number of patients enrolled per active trial site when compared to “established” markets:
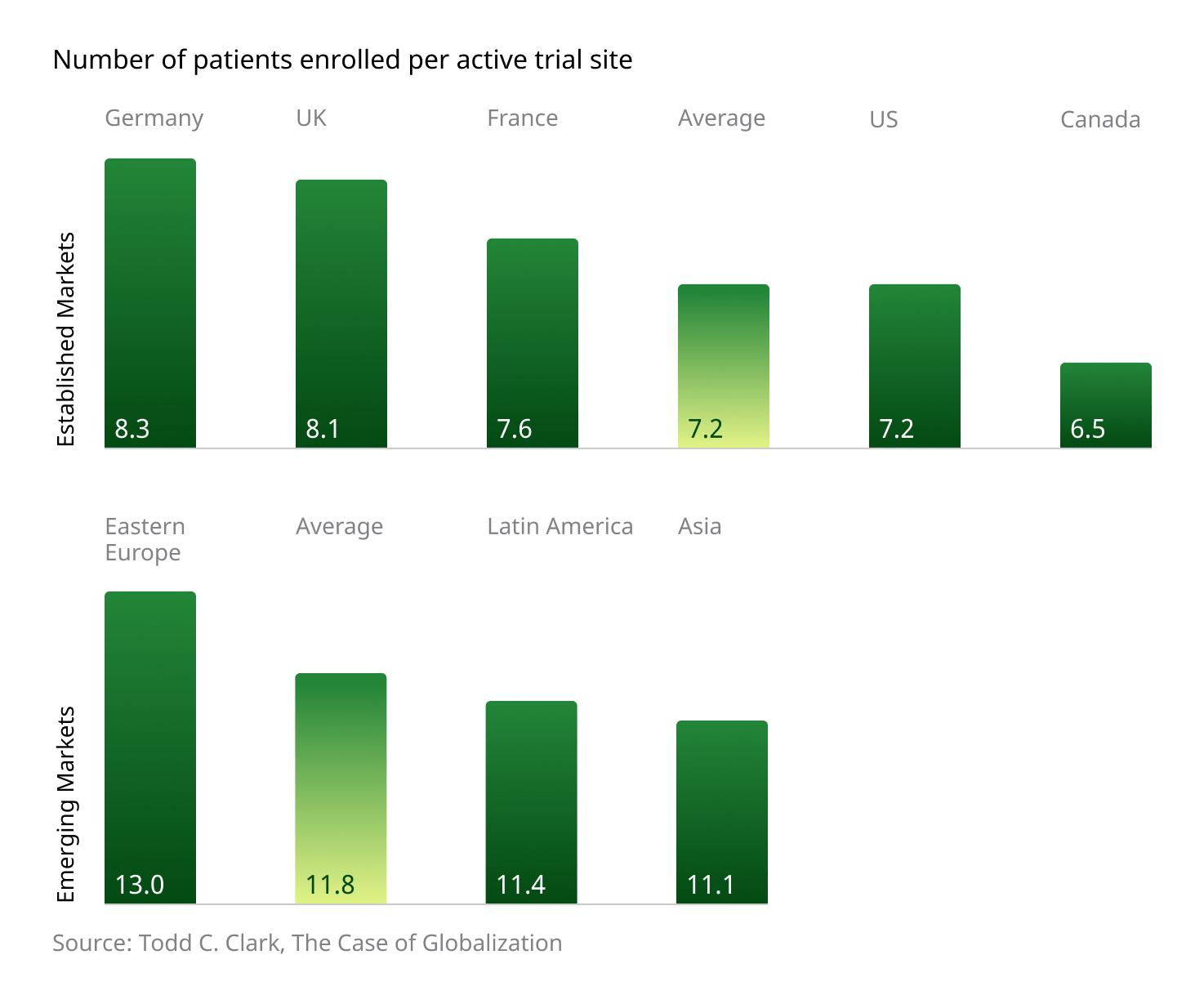
Several factors contribute to these impressive and important numbers. Local investigators are typically enthusiastic about taking a hands-on approach to new trials, and patients display a high degree of confidence in Western treatments and medical philosophies. Additionally, government-driven, centralized healthcare systems help concentrate patients and ensure a high degree of administrative consistency, with regulatory bodies supporting joint participation between public and private entities in the pursuit of advancing innovative global medicine.
Superior Patient Retention, Data Quality, and FDA Audit Results
A high degree of consumer trust in the patient-physician relationships helps the CEE region boast a high patient selection rate, and subsequently, retention rate. With so many applicants signing up, physicians can select the best, most reliable patients for enrolment, increasing long-term consistency.
“Central and Eastern Europe has accounted for 50% of the Phase II and III patients from Europe (Wyeth data) with a dropout rate of less than 5% compared to 20% in Western Europe.”
Improved patient retention is likely a key factor behind the region’s consistently high data quality. The CEE region has historically received fewer data queries and major findings on Sponsor audits when compared to Western European counterparts. Additionally, FDA audit results tend to be better when compared to trials conducted in the US or Western Europe, with CEE protocols granting investigators full access to source documents containing verifiable clinical data.
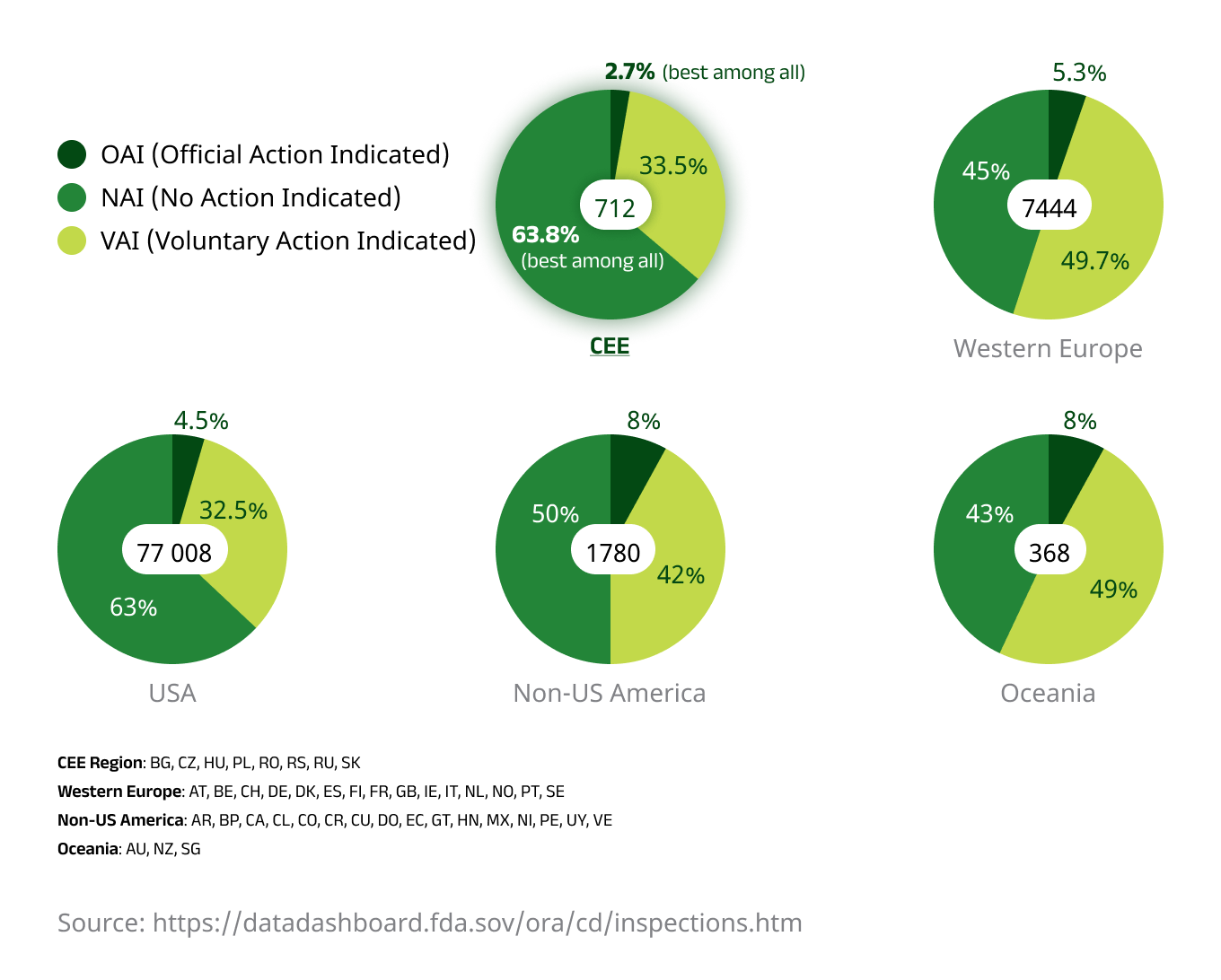
An illustration showing FDA inspection activity during its Bioresearch Monitoring initiative from 2008 to 2022, with the CEE region demonstrating the lowest level of case escalation.
Qualified, Motivated Investigators and Monitors
Local investigators are incentivized to deliver excellent service in several ways. First, in this region, the investigator fee represents a larger percentage of total compensation, tying compensation to performance and incentivizing attention to detail. Additionally, working with US, EU, and Asia-Pacific-based firms is considered a sign of merit, attracting capable, qualified talent looking to work with world-class companies.
A strong affiliation with academic and industrial training programs also works to your benefit, attracting a wide talent pool that includes qualified physicians capable of serving as CRAs or project managers. When compared to Western Europe, more practitioners are employed by teaching hospitals and university clinics, further widening your prospective talent pool. Finally, but importantly, investigators are experienced in using EDC systems for clinical trials, communicating efficiently with English-language helpdesks, and reducing the overall support burden required to align and work with CRAs.
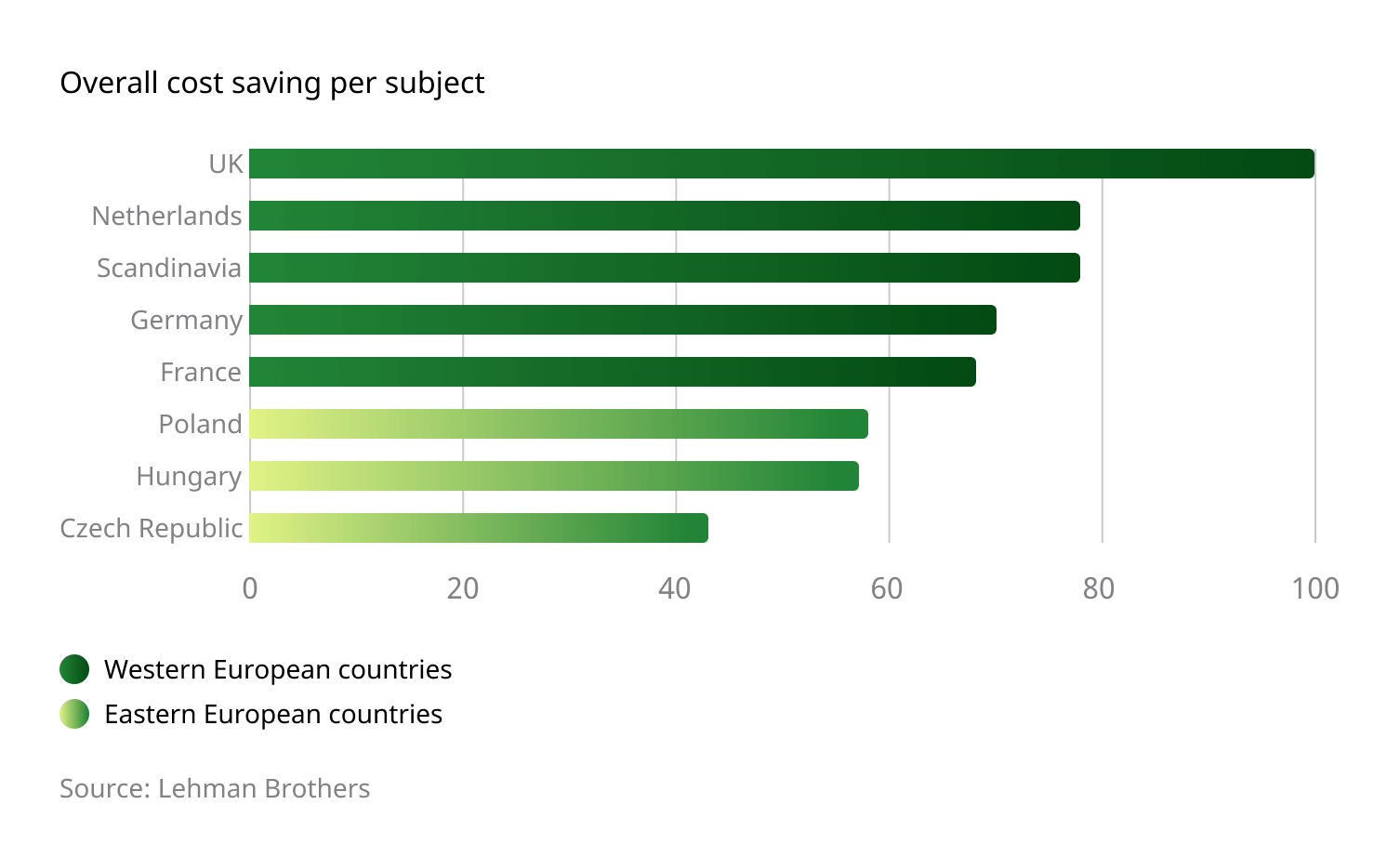
Remarkable Cost Savings
The investigation and monitoring fees associated with conducting a clinical trial in Eastern Europe are appreciably lower than those encountered in Western Europe and the US. Monitoring costs are typically 60-70% of US costs, with investigator and hospital fees remaining well below the 70% in a vast majority of projects. Because of the centralized operational and administrative structures employed by the region, additional, out-of-pocket costs – such as travel, accommodations, and postage – are also significantly lower.