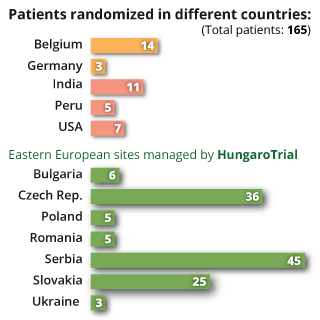
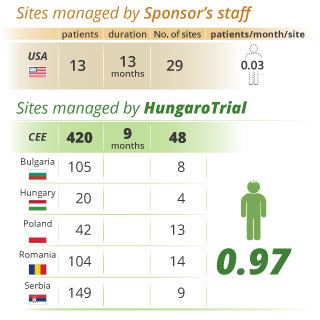
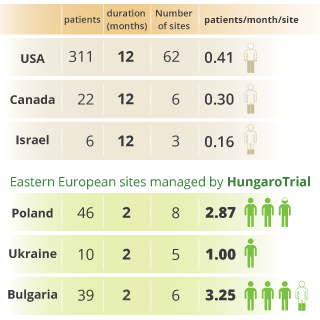
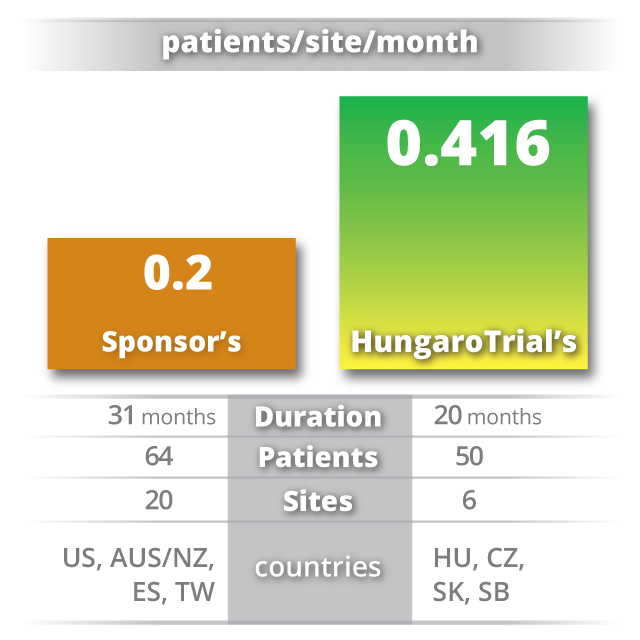
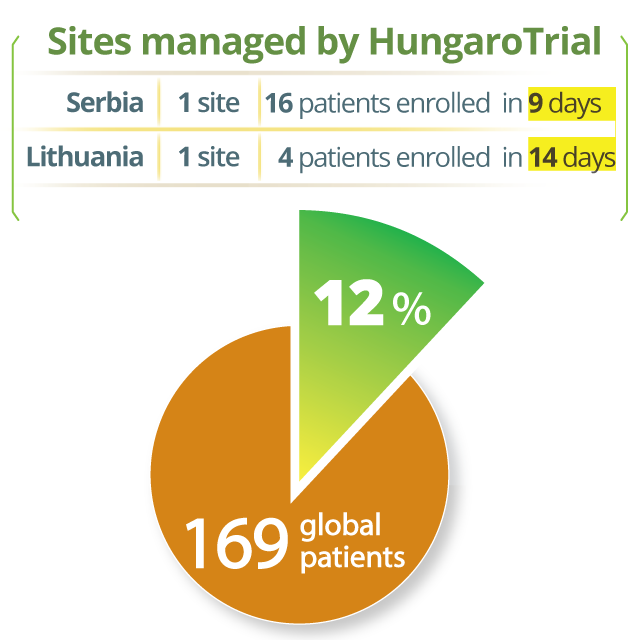
Fast study start up and patient recruitment




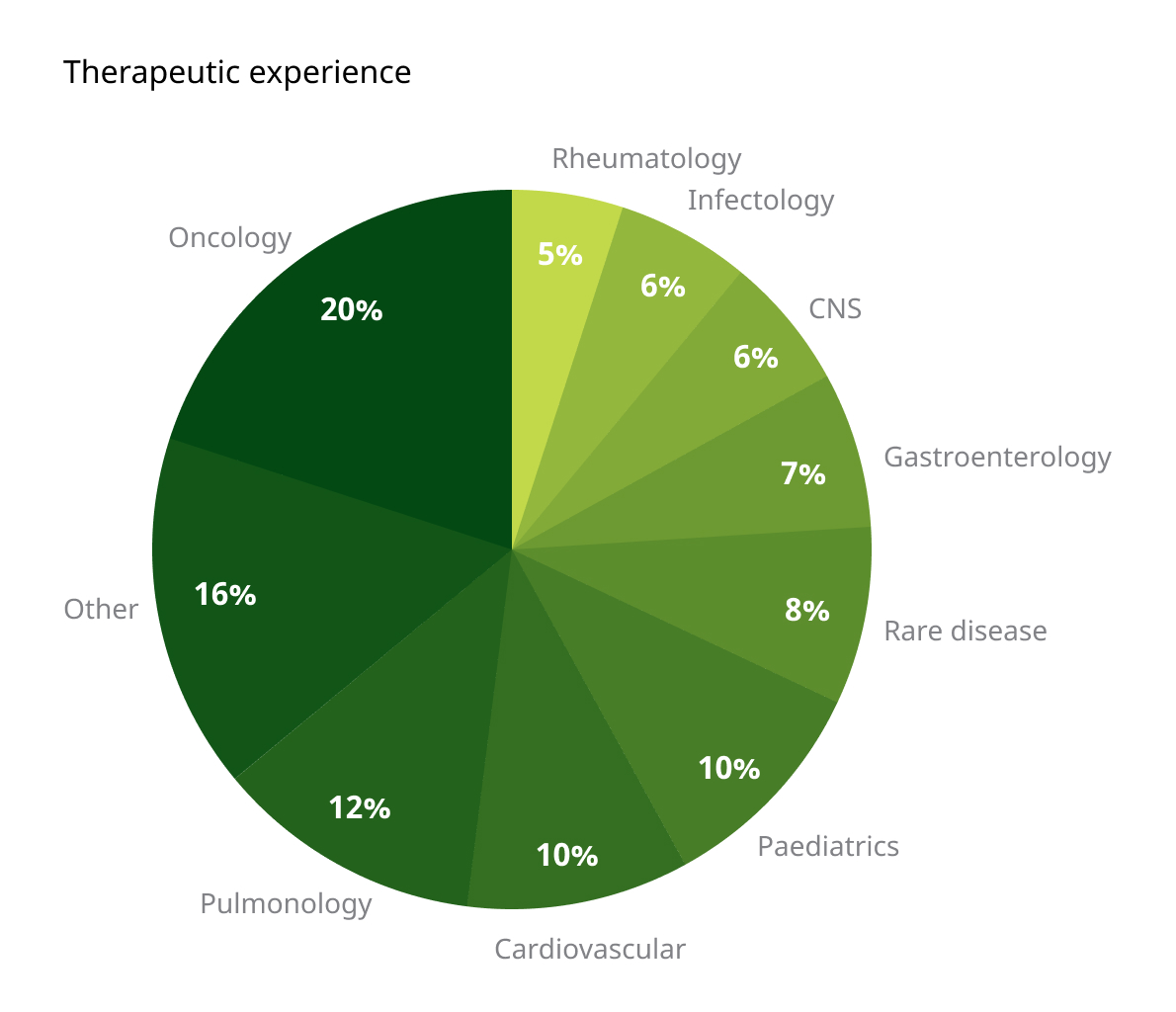
Therapeutic areas
We offer clinical research services across a broad spectrum of therapeutic areas, including Oncology, Respiratory, Cardiovascular, CNS, Rare disease, Infectiology and many others. Our experience lets us facilitate the complex clinical trials and provide tailored solutions for pharmaceutical and biotechnology companies.
Detailed experience list
Example in key development areas

For over 20 years, oncology clinical trials have comprised HungaroTrial’s largest area of expertise. We have provided services for 50+ studies in the Central and Eastern European region. In 2008, we created a highly specialized Oncology Group comprised of Regulatory Specialists and project managers with valuable Oncology experience.

Whether your indication has millions of potential participants across the globe, or just dozens, know that HungaroTrial’s dedicated team, as well as our global network of trusted Investigators and clinical trial sites, can offer the resources and expertise you need to succeed.

We have more than two decades of experience in cardiovascular research and have built a world-class Investigator Network that includes 80+ Investigators all over the CEE. We offer specialty expertise and proven solutions for Sponsor companies developing drugs and devices in Cardiovascular indications.

To be successful, rare disease studies need to be designed and run with a deep understanding of pathway of care and disease nuances. At HungaroTrial, our well-trained and experienced team can create solutions to meet any challenge across multiple rare disease indications.
For further information about our experience in other therapeutic areas
Phase I. clinical trials
HungaroTrial has extensive experience facilitating single or multi-center phase I. clinical trials. We have partnered with Central and Eastern Europe’s leading Phase I. Units to help companies assess the following properties across medically diverse patient populations:
- Pharmacokinetics
- Pharmacodynamics
- Drug-drug interactions
- Bioequivalence (in close partnership with three clinical pharmacologic units, we provide independent, experienced monitoring services)
- Bioavailability
- Dose Finding
- Cardiovascular Risk Assessment
Throughout the entire process, we pay close attention to the safety of trial subjects by setting up and managing Data Safety Monitoring Board (DSMB) operations. We ensure optimal data quality through close monitoring by Senior CRA staff.



Phase II-III. clinical trials
HungaroTrial excels at study design, planning, and executional support for Phase II-III. clinical trials:
- Senior staff, comprised of experienced full-time physicians, provides strategic direction throughout the entire process, ensuring your study proceeds with the rigor needed to meet key clinical and regulatory imperatives
- Long-standing relationships with regional academic and clinical opinion leaders ensure we bring the right perspective to every project
- Ongoing, close collaboration with high-volume patient recruitment sites – both academic- and independent-affiliated – help us ensure fast enrolment and start-up without sacrificing quality
- Rigorous and routine training keeps our Clinical Team’s working knowledge as contemporary and valuable as possible
Beyond clinical and medical operations, our dedicated Regulatory Team helps customers navigate complex and dynamic regulatory landscapes:
- We stay abreast of the most current regulations, notices, and regional events to ensure your trial documentation makes an excellent impression when it matters most
- Locally based Regulatory Officers help optimize clinical trial approval time in each country of operation
- Team members possess deep experience in DSMB management

Rescue trials
HungaroTrial has a rich history facilitating successful rescue trial sites in the CEE region. We help our partners save time, resources, and stress by leveraging our infrastructure, relationships, and experience to achieve results quickly and reliably.
Here are just some of the many examples we can proudly speak to:


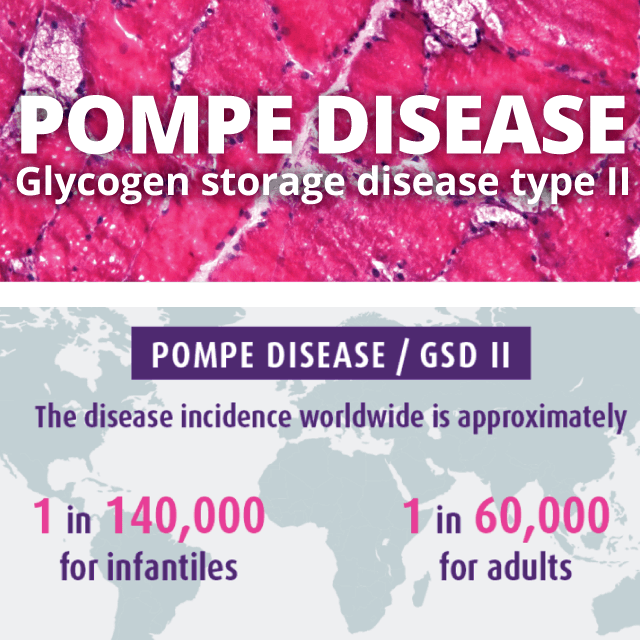
Rare disease studies
Successful management of rare disease clinical trials requires both a nuanced understanding of established protocols, as well as the ability to pilot novel approaches to day-to-day operations. At HungaroTrial, we have a strong track record of helping rare disease drug candidates reach regulatory approval.

Post-marketing trials
HungaroTrial’s expert team can effectively support the planning, design, implementation, and evaluation of post-marketing clinical programs. We have successfully supported several post-marketing study initiatives in the past, including in the Therapeutic areas.
For more information on our work in this area, please contact the HungaroTrial Team.

Advance Your Program Today
HungaroTrial is ready to help you complete your clinical trial program on time and to the highest standards.