Patient recruitment strategies in clinical trials
CEE specialist
HungaroTrial’s rich legacy in the Central and Eastern European (CEE) region makes us the premiere choice for pharmaceutical and biotechnology companies seeking fast, and valuable clinical trials services within the region. Our local heritage, experience, clinical and regulatory relationships, and professional network has helped us deliver outstanding results for hundreds of life science companies with products in highly diverse specialties.




Whether you need to find the best trial sites for your study, navigate complex and ever-evolving regulatory landscapes, or align IP importation, storage, and distribution, we can help you meet your most crucial objectives in a timely, resource-conscious manner.
Our local specialists have years of working experience with the leading clinical trial sites and key opinion leaders (KOLs) in each region, as well as with important service providers, including laboratories, IP distributors or home nursing services.
Additionally, our dedicated Regulatory Team stays informed of clinical trial regulations and application procedures in all countries of operation, regularly updating Clinical Staff and creating a robust, reliable value chain from project start to completion.
At every step, HungaroTrial curates a dedicated white glove experience. By serving as a partner instead of a transactional, we factor your operational and strategic nuances, helping you achieve demonstrable success and laying the groundwork for effective product development.

Fast study start up and patient enrolment
Fast and rigorous study start-up and patient enrolment is crucial to meeting your organizational goals while staying resource efficient. Slow enrolment and start-up have significant financial and operational consequences, including missed timelines and inflated budgets. Additionally, if this key component is not handled with care, additional challenges can arise at the point of regulatory approval, leading to further consequences and costs.
Rapid patient enrolment and study start-up is one of HungaroTrial’s core competencies, both in standard and rescue trial situations. By using our knowledge and resources to rigorously assess and select sites, we reduce start-up friction and accelerate results.
When we are engaged for a clinical trial project, every site in our database is evaluated thoroughly for past performance as it relates to the project’s specific target indication. We tap the perspective of our dedicated Project Managers to gain first-hand knowledge of the site’s performance, working quality, responsiveness, and cooperation.
After study initiation, HungaroTrial applies special enrolment management techniques developed and optimized over the course of our two decades serving the CEE region. These techniques include Pre-screening, Advertisement deployment, Referral Network recruitment, and coordinating alongside Recruitment Agencies.
Meanwhile, our Regulatory Team chooses the optimal regulatory route based on your product and target indication. They compile necessary submission documentation using a rigorous, multistep QC process, and manage timely responses to questions posed by relevant Competent Authorities.
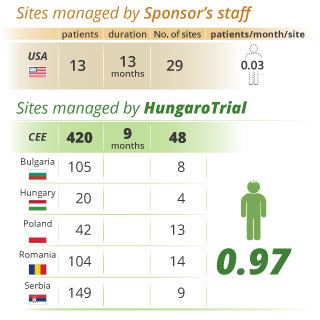
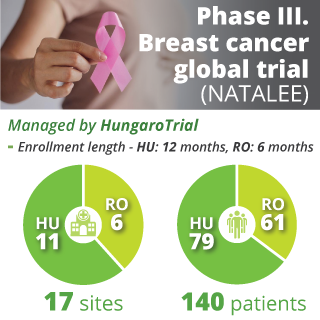
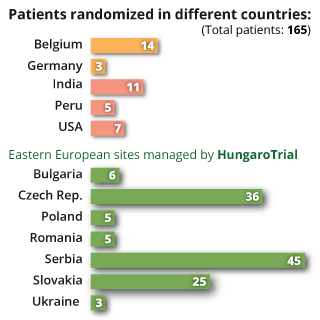
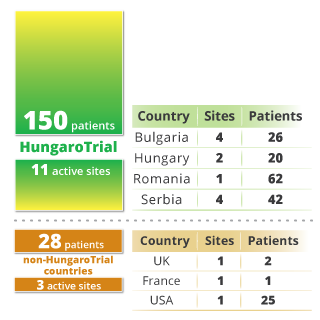
As a result of our unique, highly dedicated approach to patient enrolment, HungaroTrial has proudly achieved a higher patient enrolment rate in the CEE region than competing firms. Below, you will find several case studies illustrating how our competencies and service-minded execution drive real-world success.


Focus on patient safety
Patient safety is an absolute priority for every member of the HungaroTrial team, and this is evident in our operational model.
Prior to initiating patient enrolment, we develop and share a Safety Management Plan that is reviewed and approved by our experienced Senior Staff Members, which includes clinicians and operational experts.
Our CRA training programs focus heavily on patient safety, and our Project Managers have significant experience in setting up and working with a Data and Safety Monitoring Board (DSMB) for periodic review of safety data throughout your study.
We train more Medical Monitors than any other firm in the region and excel at developing effective medical monitoring procedures.
It is our mission to ensure the well-being and integrity of people enrolled in our studies, which is why we dedicate significant resources to creating the collaborative and educational channels required to ensure attention to safety at all points.



Success stories - Case studies
Our case studies are evidence of our unparalleled ability to provide patient recruitment and start-up results in the Central and Eastern European region, as well as our commitment to managing every project an unwavering adherence to operational and regulatory excellence.
For more case studies, contact our Team at
info@hungarotrial.com
Advance Your Program Today
HungaroTrial is ready to help you complete your clinical trial program on time and to the highest standards.